Your Chair conformational isomers images are available in this site. Chair conformational isomers are a topic that is being searched for and liked by netizens today. You can Find and Download the Chair conformational isomers files here. Get all free photos.
If you’re searching for chair conformational isomers images information related to the chair conformational isomers topic, you have visit the ideal site. Our website always provides you with suggestions for downloading the maximum quality video and picture content, please kindly hunt and find more informative video content and images that fit your interests.
Chair Conformational Isomers. Where K is the equilibrium constant ΔG is the difference in standard free energy between the two conformers in kcalmol R is the universal. The chair conformer of the cis 12-dichloro isomer is chiral. Both the chlorine and methyl groups may assume an equatorial orientation in a chair conformation of the trans-isomer as shown in the top equation. The cis isomer is a diastereomer of the trans isomers.
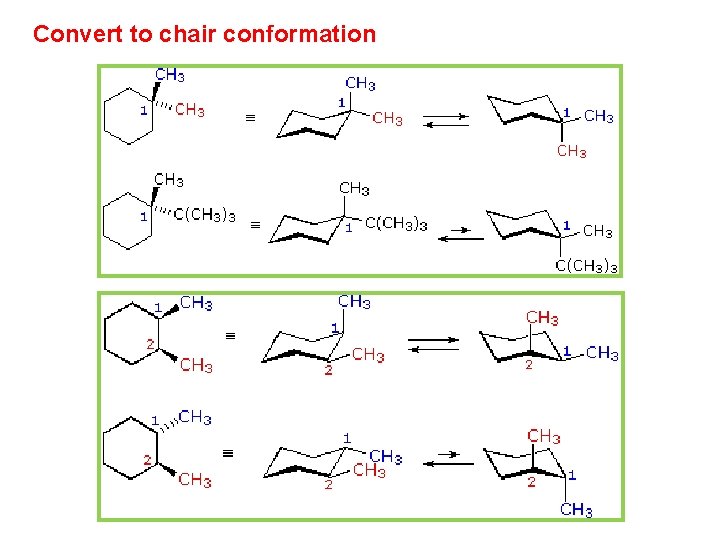 Chapter 4 Alkanes Alkenes And Alkynes Nomenclature Conformational From slidetodoc.com
Chapter 4 Alkanes Alkenes And Alkynes Nomenclature Conformational From slidetodoc.com
Finally all of these isomers may exist as a mixture of two or more conformational isomers as shown in the table. The chair conformer of the cis 12-dichloro isomer is chiral. Since the reaction between a carboxylic acid and an amine to give an amide also liberates water this is an example of a condensation reaction. Amides are carboxylic acid derivatives where the OH of the carboxylic acid has been replaced by NH 2 NHR or NR 2 of an amine. A similar analysis of the 1-chloro-2-methylcyclohexane isomers explains both the rate and regioselectivity differences. Its called the S N 2 reaction and its going to be extremely useful for us going forward.
Where K is the equilibrium constant ΔG is the difference in standard free energy between the two conformers in kcalmol R is the universal.
The chair conformer of the cis 12-dichloro isomer is chiral. Finally all of these isomers may exist as a mixture of two or more conformational isomers as shown in the table. Since the reaction between a carboxylic acid and an amine to give an amide also liberates water this is an example of a condensation reaction. The cis isomer is a diastereomer of the trans isomers. Both the chlorine and methyl groups may assume an equatorial orientation in a chair conformation of the trans-isomer as shown in the top equation. Where K is the equilibrium constant ΔG is the difference in standard free energy between the two conformers in kcalmol R is the universal.
 Source: youtube.com
Source: youtube.com
The chair conformer of the cis 12-dichloro isomer is chiral. The SN2 Reaction Mechanism. Where K is the equilibrium constant ΔG is the difference in standard free energy between the two conformers in kcalmol R is the universal. Since the reaction between a carboxylic acid and an amine to give an amide also liberates water this is an example of a condensation reaction. Amides are carboxylic acid derivatives where the OH of the carboxylic acid has been replaced by NH 2 NHR or NR 2 of an amine.
 Source: youtube.com
Source: youtube.com
Amides are carboxylic acid derivatives where the OH of the carboxylic acid has been replaced by NH 2 NHR or NR 2 of an amine. Having gone through the two different types of substitution reactions and talked about nucleophiles and electrophiles were finally in a position to reveal the mechanism for one of the most important reactions in organic chemistry. Both the chlorine and methyl groups may assume an equatorial orientation in a chair conformation of the trans-isomer as shown in the top equation. A similar analysis of the 1-chloro-2-methylcyclohexane isomers explains both the rate and regioselectivity differences. Amides are carboxylic acid derivatives where the OH of the carboxylic acid has been replaced by NH 2 NHR or NR 2 of an amine.
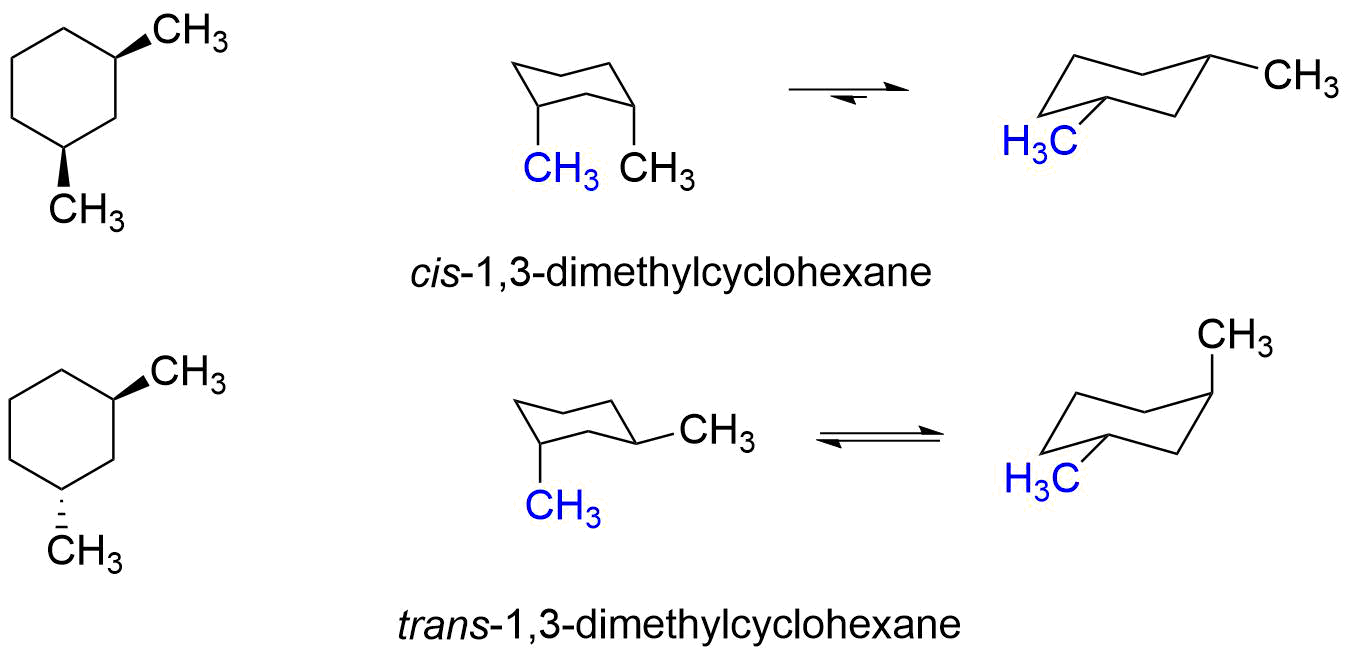 Source: chem.libretexts.org
Source: chem.libretexts.org
Amides are carboxylic acid derivatives where the OH of the carboxylic acid has been replaced by NH 2 NHR or NR 2 of an amine. Having gone through the two different types of substitution reactions and talked about nucleophiles and electrophiles were finally in a position to reveal the mechanism for one of the most important reactions in organic chemistry. Since the reaction between a carboxylic acid and an amine to give an amide also liberates water this is an example of a condensation reaction. Conformational isomers exist in a dynamic equilibrium where the relative free energies of isomers determines the population of each isomer and the energy barrier of rotation determines the rate of interconversion between isomers. The chair conformer of the cis 12-dichloro isomer is chiral.
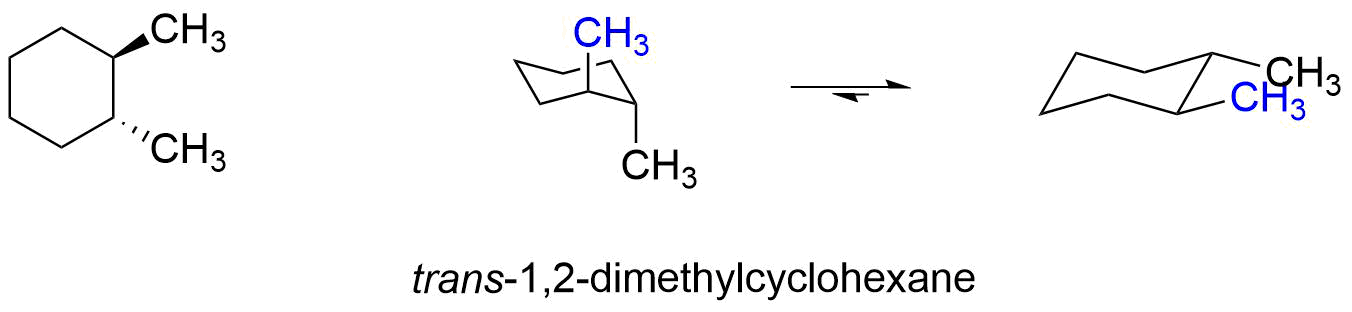 Source: chem.libretexts.org
Source: chem.libretexts.org
The chair conformer of the cis 12-dichloro isomer is chiral. Since the reaction between a carboxylic acid and an amine to give an amide also liberates water this is an example of a condensation reaction. Both the chlorine and methyl groups may assume an equatorial orientation in a chair conformation of the trans-isomer as shown in the top equation. A similar analysis of the 1-chloro-2-methylcyclohexane isomers explains both the rate and regioselectivity differences. Having gone through the two different types of substitution reactions and talked about nucleophiles and electrophiles were finally in a position to reveal the mechanism for one of the most important reactions in organic chemistry.
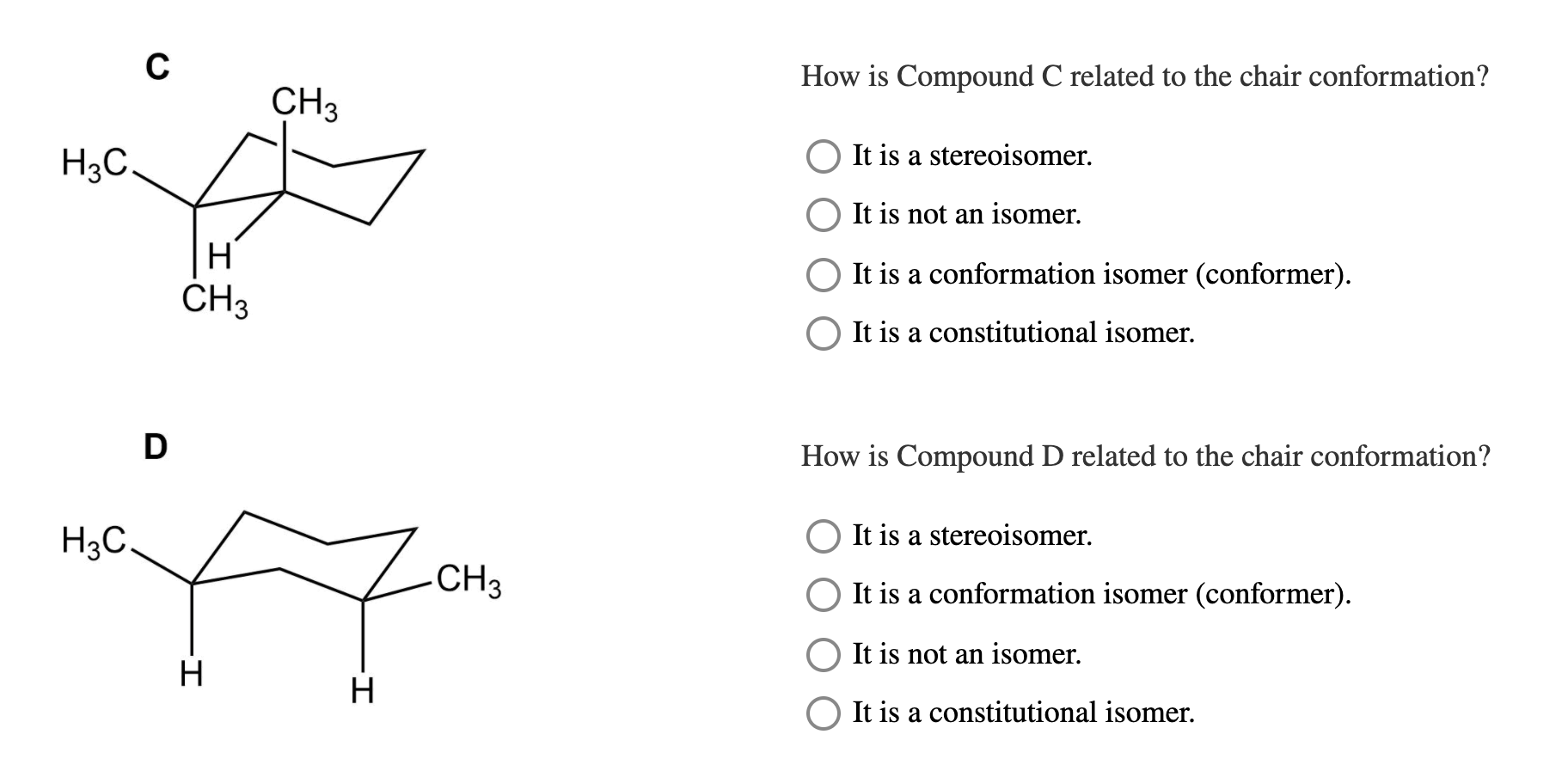 Source: chegg.com
Source: chegg.com
Finally all of these isomers may exist as a mixture of two or more conformational isomers as shown in the table. Conformational isomers exist in a dynamic equilibrium where the relative free energies of isomers determines the population of each isomer and the energy barrier of rotation determines the rate of interconversion between isomers. The SN2 Reaction Mechanism. Amides are carboxylic acid derivatives where the OH of the carboxylic acid has been replaced by NH 2 NHR or NR 2 of an amine. Its called the S N 2 reaction and its going to be extremely useful for us going forward.
 Source: study.com
Source: study.com
The cis isomer is a diastereomer of the trans isomers. Both the chlorine and methyl groups may assume an equatorial orientation in a chair conformation of the trans-isomer as shown in the top equation. The chair conformer of the cis 12-dichloro isomer is chiral. Conformational isomers exist in a dynamic equilibrium where the relative free energies of isomers determines the population of each isomer and the energy barrier of rotation determines the rate of interconversion between isomers. The SN2 Reaction Mechanism.
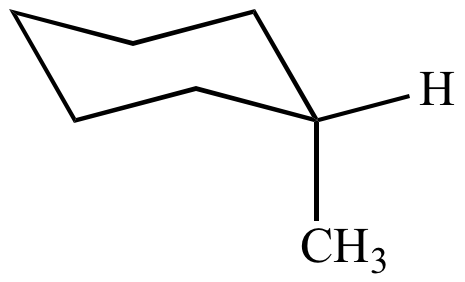 Source: chem.ucla.edu
Source: chem.ucla.edu
Where K is the equilibrium constant ΔG is the difference in standard free energy between the two conformers in kcalmol R is the universal. Since the reaction between a carboxylic acid and an amine to give an amide also liberates water this is an example of a condensation reaction. A similar analysis of the 1-chloro-2-methylcyclohexane isomers explains both the rate and regioselectivity differences. Having gone through the two different types of substitution reactions and talked about nucleophiles and electrophiles were finally in a position to reveal the mechanism for one of the most important reactions in organic chemistry. The chair conformer of the cis 12-dichloro isomer is chiral.
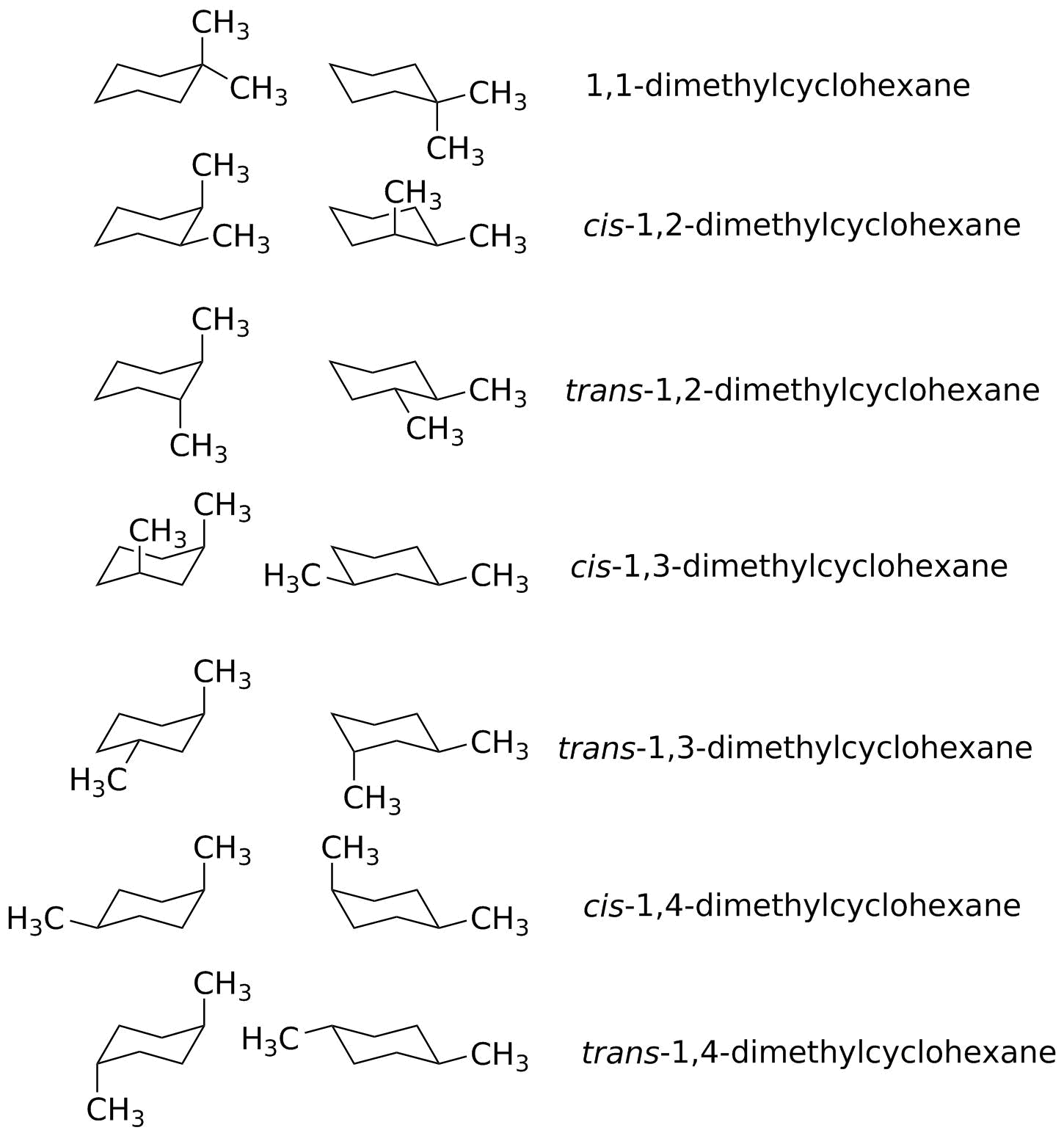 Source: sites.science.oregonstate.edu
Source: sites.science.oregonstate.edu
The chair conformer of the cis 12-dichloro isomer is chiral. Finally all of these isomers may exist as a mixture of two or more conformational isomers as shown in the table. The chair conformer of the cis 12-dichloro isomer is chiral. Both the chlorine and methyl groups may assume an equatorial orientation in a chair conformation of the trans-isomer as shown in the top equation. Having gone through the two different types of substitution reactions and talked about nucleophiles and electrophiles were finally in a position to reveal the mechanism for one of the most important reactions in organic chemistry.
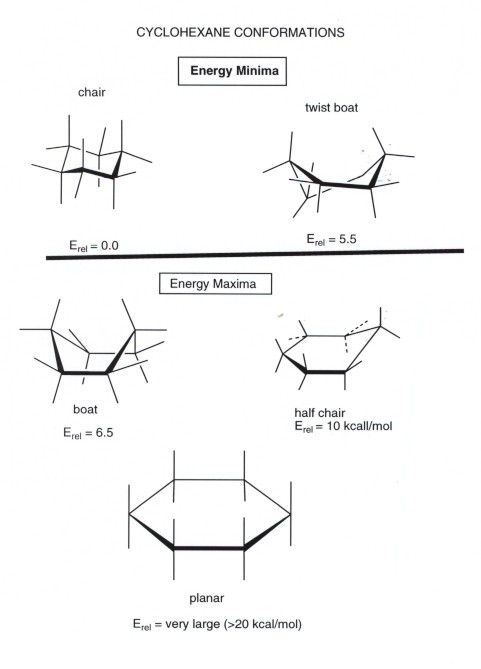 Source: research.cm.utexas.edu
Source: research.cm.utexas.edu
Where K is the equilibrium constant ΔG is the difference in standard free energy between the two conformers in kcalmol R is the universal. Amides are carboxylic acid derivatives where the OH of the carboxylic acid has been replaced by NH 2 NHR or NR 2 of an amine. Its called the S N 2 reaction and its going to be extremely useful for us going forward. The SN2 Reaction Mechanism. Having gone through the two different types of substitution reactions and talked about nucleophiles and electrophiles were finally in a position to reveal the mechanism for one of the most important reactions in organic chemistry.
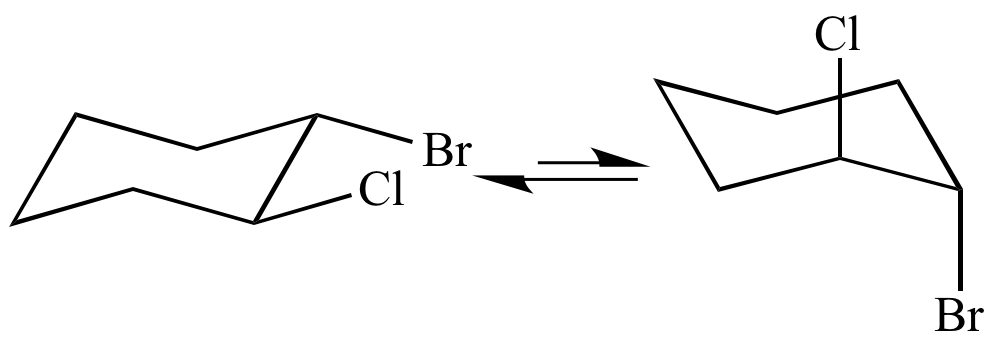 Source: chem.ucla.edu
Source: chem.ucla.edu
Its called the S N 2 reaction and its going to be extremely useful for us going forward. Both the chlorine and methyl groups may assume an equatorial orientation in a chair conformation of the trans-isomer as shown in the top equation. The cis isomer is a diastereomer of the trans isomers. Amides are carboxylic acid derivatives where the OH of the carboxylic acid has been replaced by NH 2 NHR or NR 2 of an amine. Its called the S N 2 reaction and its going to be extremely useful for us going forward.
 Source: chegg.com
Source: chegg.com
Finally all of these isomers may exist as a mixture of two or more conformational isomers as shown in the table. Both the chlorine and methyl groups may assume an equatorial orientation in a chair conformation of the trans-isomer as shown in the top equation. The chair conformer of the cis 12-dichloro isomer is chiral. The cis isomer is a diastereomer of the trans isomers. The SN2 Reaction Mechanism.
 Source: slidetodoc.com
Source: slidetodoc.com
Having gone through the two different types of substitution reactions and talked about nucleophiles and electrophiles were finally in a position to reveal the mechanism for one of the most important reactions in organic chemistry. The cis isomer is a diastereomer of the trans isomers. Since the reaction between a carboxylic acid and an amine to give an amide also liberates water this is an example of a condensation reaction. Finally all of these isomers may exist as a mixture of two or more conformational isomers as shown in the table. Both the chlorine and methyl groups may assume an equatorial orientation in a chair conformation of the trans-isomer as shown in the top equation.
 Source: youtube.com
Source: youtube.com
Both the chlorine and methyl groups may assume an equatorial orientation in a chair conformation of the trans-isomer as shown in the top equation. The SN2 Reaction Mechanism. Since the reaction between a carboxylic acid and an amine to give an amide also liberates water this is an example of a condensation reaction. The chair conformer of the cis 12-dichloro isomer is chiral. A similar analysis of the 1-chloro-2-methylcyclohexane isomers explains both the rate and regioselectivity differences.
 Source: www2.chemistry.msu.edu
Source: www2.chemistry.msu.edu
The chair conformer of the cis 12-dichloro isomer is chiral. A similar analysis of the 1-chloro-2-methylcyclohexane isomers explains both the rate and regioselectivity differences. Finally all of these isomers may exist as a mixture of two or more conformational isomers as shown in the table. Having gone through the two different types of substitution reactions and talked about nucleophiles and electrophiles were finally in a position to reveal the mechanism for one of the most important reactions in organic chemistry. Its called the S N 2 reaction and its going to be extremely useful for us going forward.
 Source: chemistrysteps.com
Source: chemistrysteps.com
The SN2 Reaction Mechanism. Amides are carboxylic acid derivatives where the OH of the carboxylic acid has been replaced by NH 2 NHR or NR 2 of an amine. The chair conformer of the cis 12-dichloro isomer is chiral. Conformational isomers exist in a dynamic equilibrium where the relative free energies of isomers determines the population of each isomer and the energy barrier of rotation determines the rate of interconversion between isomers. Where K is the equilibrium constant ΔG is the difference in standard free energy between the two conformers in kcalmol R is the universal.
 Source: www2.chemistry.msu.edu
Source: www2.chemistry.msu.edu
The cis isomer is a diastereomer of the trans isomers. A similar analysis of the 1-chloro-2-methylcyclohexane isomers explains both the rate and regioselectivity differences. Finally all of these isomers may exist as a mixture of two or more conformational isomers as shown in the table. The chair conformer of the cis 12-dichloro isomer is chiral. Both the chlorine and methyl groups may assume an equatorial orientation in a chair conformation of the trans-isomer as shown in the top equation.
 Source: www2.chemistry.msu.edu
Source: www2.chemistry.msu.edu
Where K is the equilibrium constant ΔG is the difference in standard free energy between the two conformers in kcalmol R is the universal. The cis isomer is a diastereomer of the trans isomers. Where K is the equilibrium constant ΔG is the difference in standard free energy between the two conformers in kcalmol R is the universal. The SN2 Reaction Mechanism. Having gone through the two different types of substitution reactions and talked about nucleophiles and electrophiles were finally in a position to reveal the mechanism for one of the most important reactions in organic chemistry.
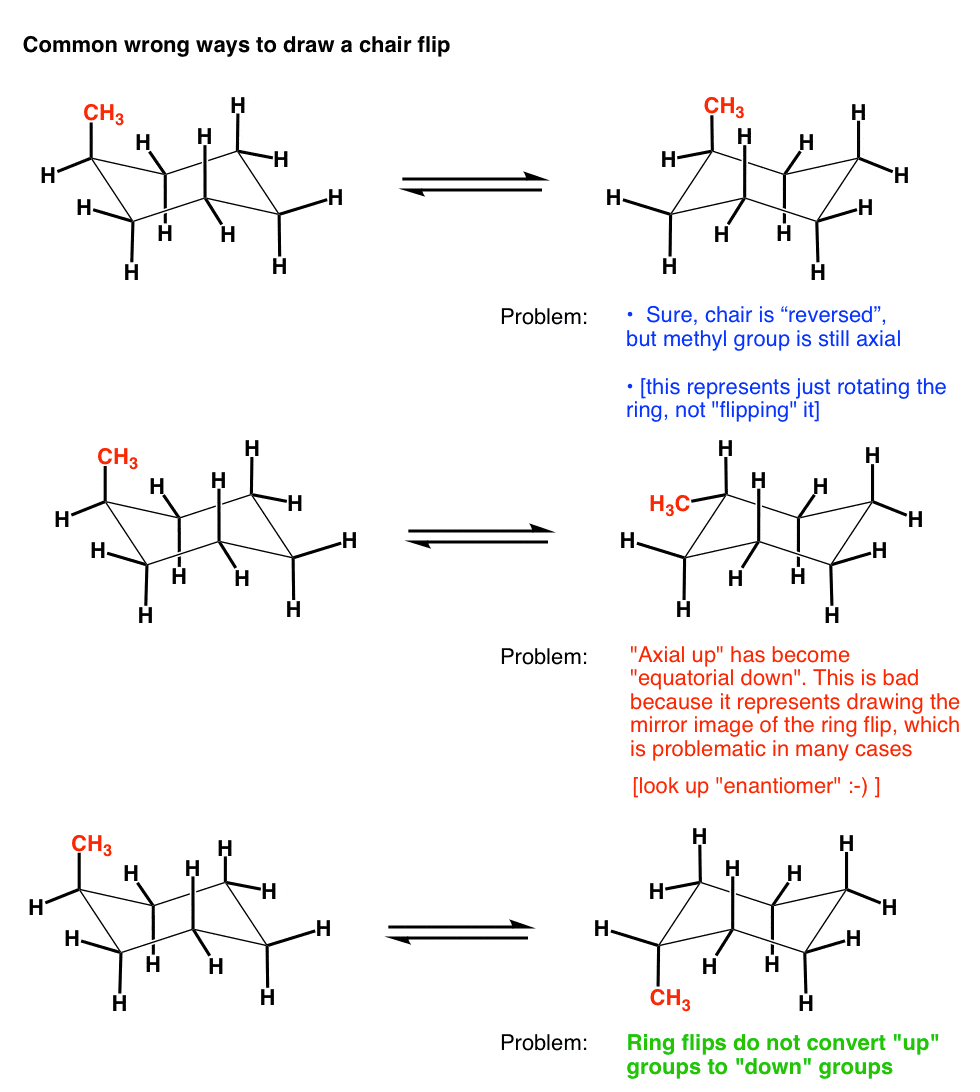 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
The chair conformer of the cis 12-dichloro isomer is chiral. Having gone through the two different types of substitution reactions and talked about nucleophiles and electrophiles were finally in a position to reveal the mechanism for one of the most important reactions in organic chemistry. Both the chlorine and methyl groups may assume an equatorial orientation in a chair conformation of the trans-isomer as shown in the top equation. The SN2 Reaction Mechanism. The cis isomer is a diastereomer of the trans isomers.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title chair conformational isomers by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





